Gas Laws in Real World Reading Assignment
Related Pages
Solving Gas Constabulary Problems
Loftier School Chemistry
Chemistry Lessons
The following table gives the Gas Law Formulas. Scroll downwards the page for more examples and solutions on how to use the Boyle's Police, Charles'Police force, Gay-Lussac's Law, Combined Gas Constabulary and Ideal Gas Law. 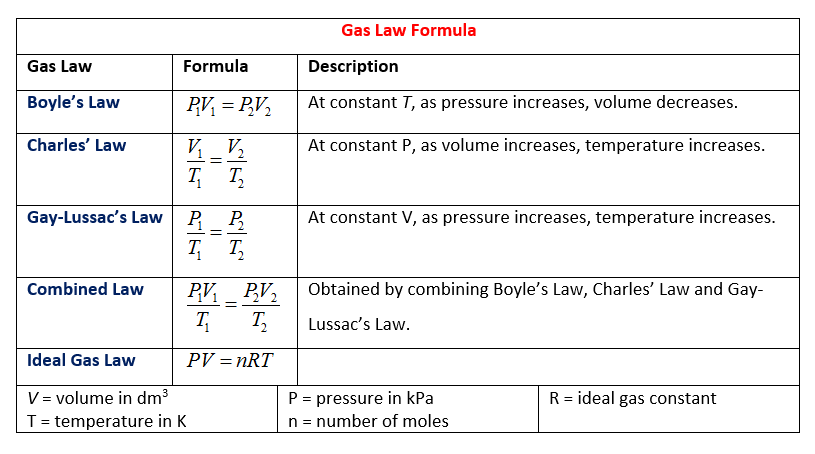
Boyle's Law
Boyle's Police states that volume of a given amount of gas held at a constant temperature varies inversely the with force per unit area. The relationship between pressure and volume of Boyle's Constabulary is expressed in mathematical terms as P1V1= Pii5ii.
An Introduction To The Relationship Betwixt Pressure And Book
A lesson on how to solve gas problems with Boyle's Law.
Example:
At 1.70 atm, a sample of gas takes up iv.25L. If the pressure in the gas is increased to 2.twoscore atm, what volition the new volume be?
- Show Video Lesson
Understanding And Applying Boyle'southward Law
Case:
A sample of Ne gas occupies 0.220L at 0.86 atm. What volition exist its volume at 29.4kPa?
- Show Video Lesson
Charles' Law
Charles' Constabulary states that the volume of a given mass of a gas is directly proportional to its Kelvin temperature at constant pressure. In mathematical terms, the relationship betwixt temperature and volume is expressed equally Fivei/T1=5ii/T2.
What Is The Relationship Between Volume And Temperature Of A Gas
A lesson on how to solve bug using Charles' Law.
Example:
A balloon takes upward 625L at 0°C. If information technology is heated to 80°C, what will its new volume exist?
- Show Video Lesson
Agreement And Applying Charles' Law
Example:
A gas at 40.0°C occupies a volume of ii.32L. If the temperature is raised to 75.0°C, what will the new volume be if the pressure is constant?
- Show Video Lesson
Gay-Lussac's Law
Gay-Lussac's Constabulary states that the pressure of a given mass of gas varies direct with the Kelvin temperature when the volume remains constant. Gay-Lussac'due south Law is expressed in a formula form every bit P1/T1 = P2/T2. When dealing with Gay-Lussac's Law, the unit of the temperature should ever be in Kelvin.
Using Gay-Lussac'due south Law To Understand The Human relationship Between A Gas' Pressure And Temperature
Example:
If the pressure in a car tire is 1.88 atm at 25°C, what will be the pressure if the temperature warms to 37°C?
- Show Video Lesson
How To Solve Word Bug That Bear witness How To Use Gay-Lussac's Constabulary?
Examples:
- The pressure in a sealed can of gas is 235kPA when it sits at room temperature (20°). If the tin can is warmed to 48°C, what will the new pressure inside the can be?
- A car tire has a pressure of 2.38 atm at 15.2°C. If the pressure within reached 4.08 atm, the tire will explode. How hot would the tire take to get for this to happen? Report the temperature in degrees Celsius.
- Testify Video Lesson
Practice Trouble to testify how to use Gay-Lussac'due south Law
Example:
In the morn, a paintball pressure level tank is at 306 atm. The weather condition heats up over the grade of the solar day, and past iii PM, the exterior temperature is roasting at 38.5°C, and the force per unit area within the tank is 324 atm. What was the temperature (in degree Celsius) in the morning?
- Show Video Lesson
Combined Gas Law
The Combined Gas Police force combines Charles' Law, Boyle's Law and Gay Lussac's Police force. The Combined Gas Police states that a gas' (pressure × volume)/temperature = abiding.
Instance:
A gas at 110kPa at 30.0°C fills a flexible container with an initial volume of 2.00L. If the temperature is raised to fourscore,0°C and the pressure increases to 440Kpa, what is the new volume?
- Show Video Lesson
How To Solve Bug With The Combined Gas Equation?
Example:
A 40.0L airship is filled with air at sea level (one.00 atm, 25.0°C). It is tied to a stone and thrown in a cold body of water, and it sinks to the point where the temperature is 4.0°C and the pressure level is 11.0 atm. What will its new volume be?
- Show Video Lesson
Platonic Gas Law
The Ideal Gas Constabulary mathematically relates the pressure, volume, amount and temperature of a gas with the equation:
pressure × volume = moles × ideal gas abiding × temperature;
PV = nRT.
The Ideal Gas Law is ideal because information technology ignores interactions between the gas particles in club to simplify the equation. There is besides a Existent Gas Law which is much more than complicated and produces a upshot which, under almost circumstances, is almost identical to that predicted by the Platonic Gas Law.
Understanding And Applying The Ideal Gas Constabulary
Example:
What is the pressure in atm of a 0.108 mol sample of the gas at a temperature of 20.0°C if its book is 0.505L?
- Evidence Video Lesson
Sample Problems For Using The Platonic Gas Law, PV = nRT
Examples:
- 2.three moles of Helium gas are at a pressure of one.70 atm, and the temperature is 41°C. What is the volume of the gas?
- At a certain temperature, 3.24 moles of CO2 gas at ii.15 atm have upwardly a colume of 35.28L. What is this temperature (in Celsius)?
- Prove Video Lesson
Try the free Mathway calculator and problem solver below to practice various math topics. Try the given examples, or type in your ain trouble and check your answer with the step-past-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback folio.
Gas Laws in Real World Reading Assignment
Source: https://www.onlinemathlearning.com/gas-laws-chemistry.html